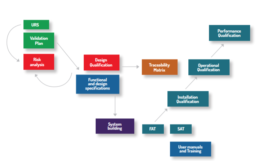
Validation in Pharma
Pharmaceutical critical processes request a high level of standardization, repeatability and safety, as
an effect of the increasing demanding level of regulatory agencies to guarantee patience safety and
treatment efficacy.
Good Manufacturing Practices (GMPs) are the collection of the applicable rules to pharma and they are
one of the driving forces of the three above mentioned targets aiming to the regulatory compliance and
manufacturing authorization.
3P has the expertise to challenge instruments, equipment and systems to demonstrate their suitability
to reach a high level of safety and quality during the medicinal products manufacturing processes and
ancillary activities by means of validation tools.
Beside GMPs, Good Automatic Manufacturing Practices (GAMPs) are pillars of the pharmaceutical
manufacturing, gathering worldwide applied guidelines as ISPE baseline® Guide, currently one of the
main reference while talking about validation.